The Pfizer-BioNTech COVID-19 vaccine tozinameran and the Moderna COVID-19 vaccine mRNA-173. MIS multisystem inflammatory syndrome.

The Race To Develop A Covid 19 Vaccine Article Nursingcenter
Severe Acute Respiratory Syndrome Coronavirus 2 SARS-CoV-2 is a new type of coronavirus that causes the Coronavirus Disease 2019 COVID-19 which has been the most challenging pandemic in this century.
Vaccination covid 19 journal. Published by Cambridge University Press on behalf of The Nutrition Society Now that a number of COVID-19 vaccines are being employed to control the current pandemic we are concerned about the likelihood of a poor response in the frail or malnourished elderly which would reduce the effectiveness of the vaccination campaigns. Timeline displaying intervals between coronavirus COVID-19 vaccine acute COVID-19 symptom onset and MIS symptom onset in patients in California USA. Chin and Others Between December 22 2020 and March 4 2021 the BNT162b2 or mRNA-1273 vaccine was.
ABOUT COVID-19 VACCINES COVID-19 vaccination efforts began nationwide in December 2020 after the US. Food and Drug Administration authorized emergency use of the Pfizer-BioNTech and Moderna vaccines. Both vaccines are messenger RNA mRNA vaccines 7 9.
The paper is entitled Informed consent disclosure to vaccine trial subjects of risk of covid-19 vaccines. The cumulative incidence of Covid-19 cases over time among placebo and vaccine recipients begins to diverge by 12 days after the first dose 7. 1-3 In late February 2021 the US Food and Drug Administration granted Emergency Use Authorization for a third COVID-19 vaccine a single-dose adenovirus vector-based vaccine.
Among the 310 million Covid-19 vaccines given several adverse events are reported at high rates in the days immediately after vaccination and then fall precipitously afterward. After the vaccines were developed and approved the. In December 2020 the US government issued emergency use authorization for two 2-dose severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 vaccines both estimated to be 94 efficacious in preventing symptomatic coronavirus disease COVID-19 13The Advisory Committee on Immunization Practices immediately recommended the prioritization of frontline workers and high.
At that moment major research efforts were being undertaken but it was not clear whether any vaccine would actually be found. Correspondence Covid-19 Vaccine Acceptance in California State Prisons ET. Since then several real-world studies have been published.
At the time this article was written two anti-COVID-19 vaccines were approved by the US. Patient 2 was a 40-year-old Hispanic man who sought care after 6. Data from an observational study of 7000 healthcare workers in Israel suggests that vaccine efficacy against symptomatic COVID-19 infection is 85 from 1528 days after the first dose.
A third vaccine Ad26COV2S is now being developed by Johnson Johnson. Developing Covid-19 Vaccines at Pandemic Speed An ideal vaccine platform would support development from viral sequencing to clinical trials in. Abstract Background As mass vaccination campaigns against coronavirus disease 2019 Covid-19 commence worldwide vaccine effectiveness needs to be assessed for a.
It is notable that as of April 26 2021 more than 100000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction 47 have enrolled in. Preferences toward vaccination against COVID-19 The vaccination module went into the field with tranches 7 to 9 in June and July 2020 and covered a total of 851 persons aged 19 years and older. Patients havent been officially warned about this problem although the evidence was published in the International Journal of Clinical Practice for October 2020.
Approximately 96 COVID-19 vaccines are at various stages of clinical development1 At present we have the interim results of four studies published in scientific journals on the PfizerBioNTech BNT162b2 mRNA vaccine2 the ModernaUS National Institutes of Health NIH mRNA-1273 vaccine3 the AstraZenecaOxford ChAdOx1 nCov-19 vaccine4 and the Gamaleya. In December 2020 2 mRNA-based COVID-19 vaccines Pfizer-BioNTech and Moderna were granted Emergency Use Authorization by the US Food and Drug Administration as 2-dose series and recommended for use by the Advisory Committee on Immunization Practices. Food and Drug Administration.
Considering its high mortality and rapid spread an effective vaccine is urgently needed to control this pandemic. As a result the academia industry and.
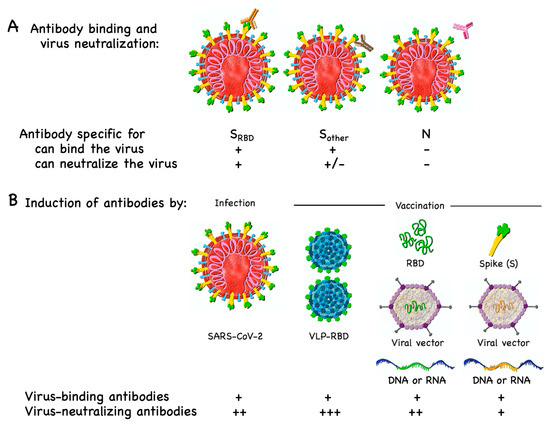
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html
Acceptance And Attitudes Toward Covid 19 Vaccines A Cross Sectional Study From Jordan
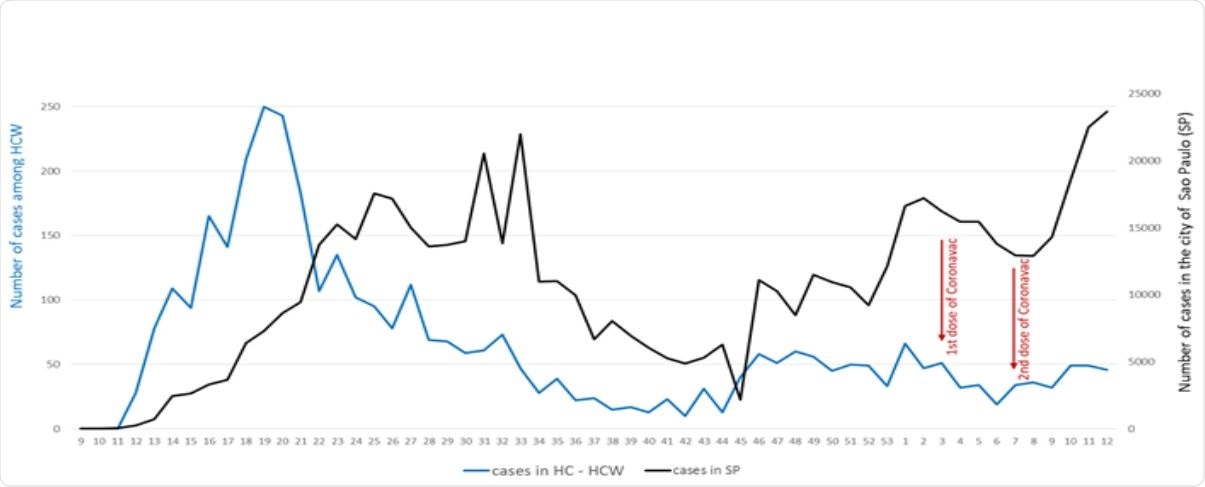
Sinovac Covid 19 Vaccine Shows 50 Effectiveness In A Cohort Of Brazilian Healthcare Workers

Covid 19 Vaccine Hesitancy And Resistance Correlates In A Nationally Representative Longitudinal Survey Of The Australian Population
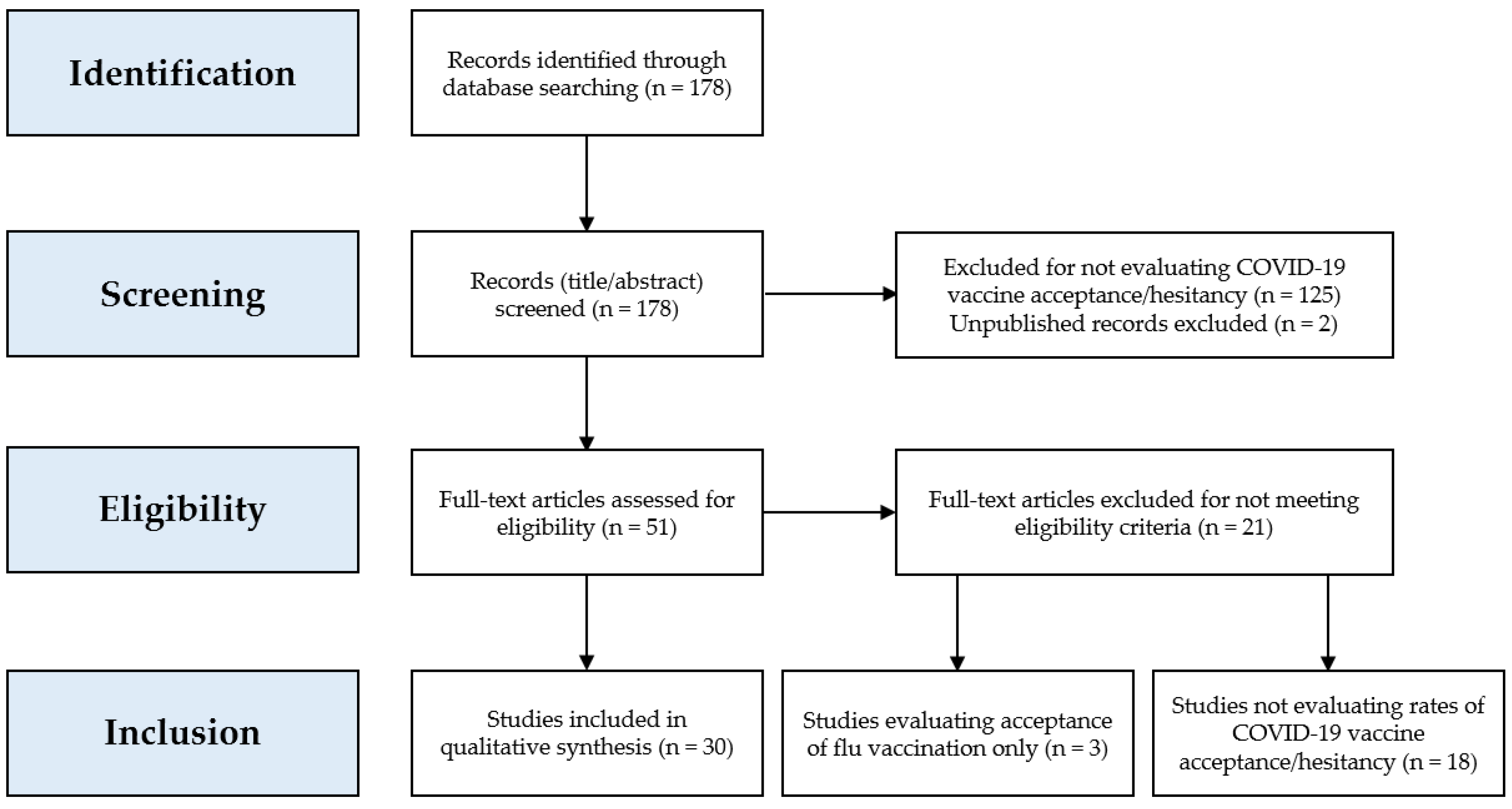
Vaccines Free Full Text Covid 19 Vaccine Hesitancy Worldwide A Concise Systematic Review Of Vaccine Acceptance Rates Html

Covid 19 Vaccine Acceptance And Its Associated Factors Among Pregnant Ijgm
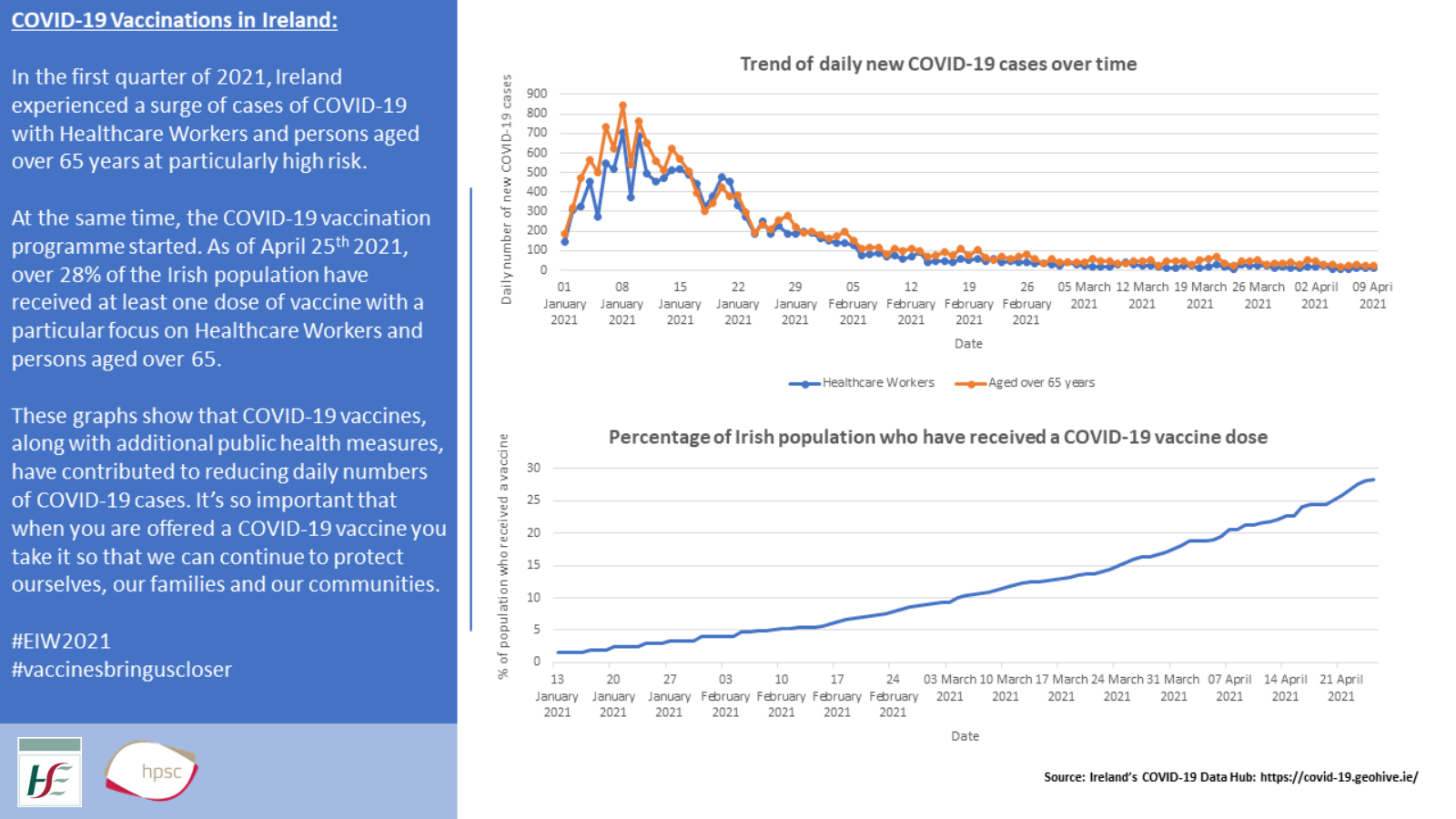
Covid 19 Vaccine Studies Hse Ie
Https Apps Who Int Iris Bitstream Handle 10665 340217 Who Euro 2021 2141 41896 57484 Eng Pdf
Covid 19 And Vaccine Hesitancy A Longitudinal Study
Plos Biology Monitor For Covid 19 Vaccine Resistance Evolution During Clinical Trials
Chinese Consumers Willingness To Get A Covid 19 Vaccine And Willingness To Pay For It
Examining The Effect Of Information Channel On Covid 19 Vaccine Acceptance

All Together The United News Brand Campaign To Tackle Covid 19
Chinese Consumers Willingness To Get A Covid 19 Vaccine And Willingness To Pay For It
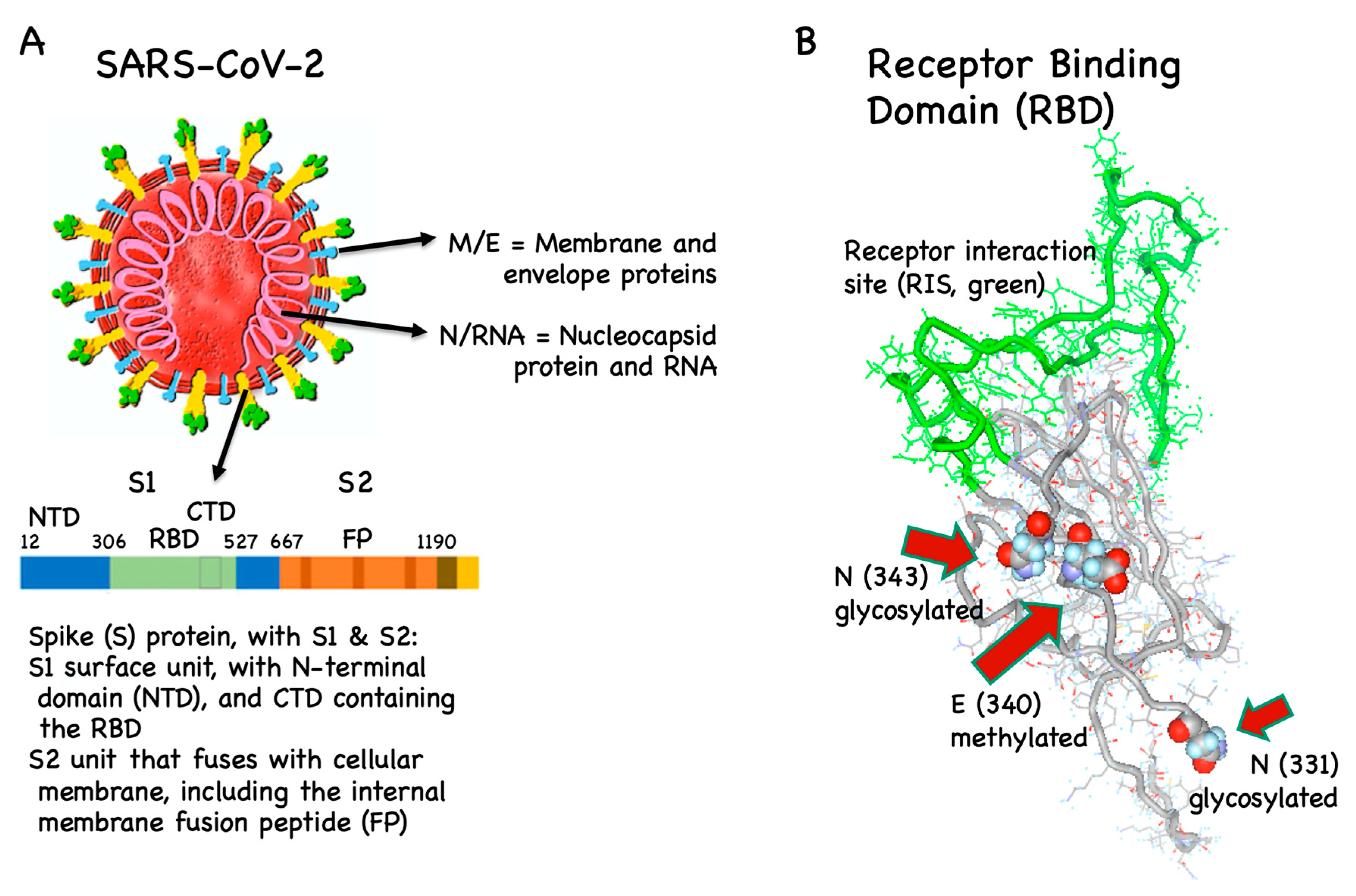
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html
Chinese Consumers Willingness To Get A Covid 19 Vaccine And Willingness To Pay For It
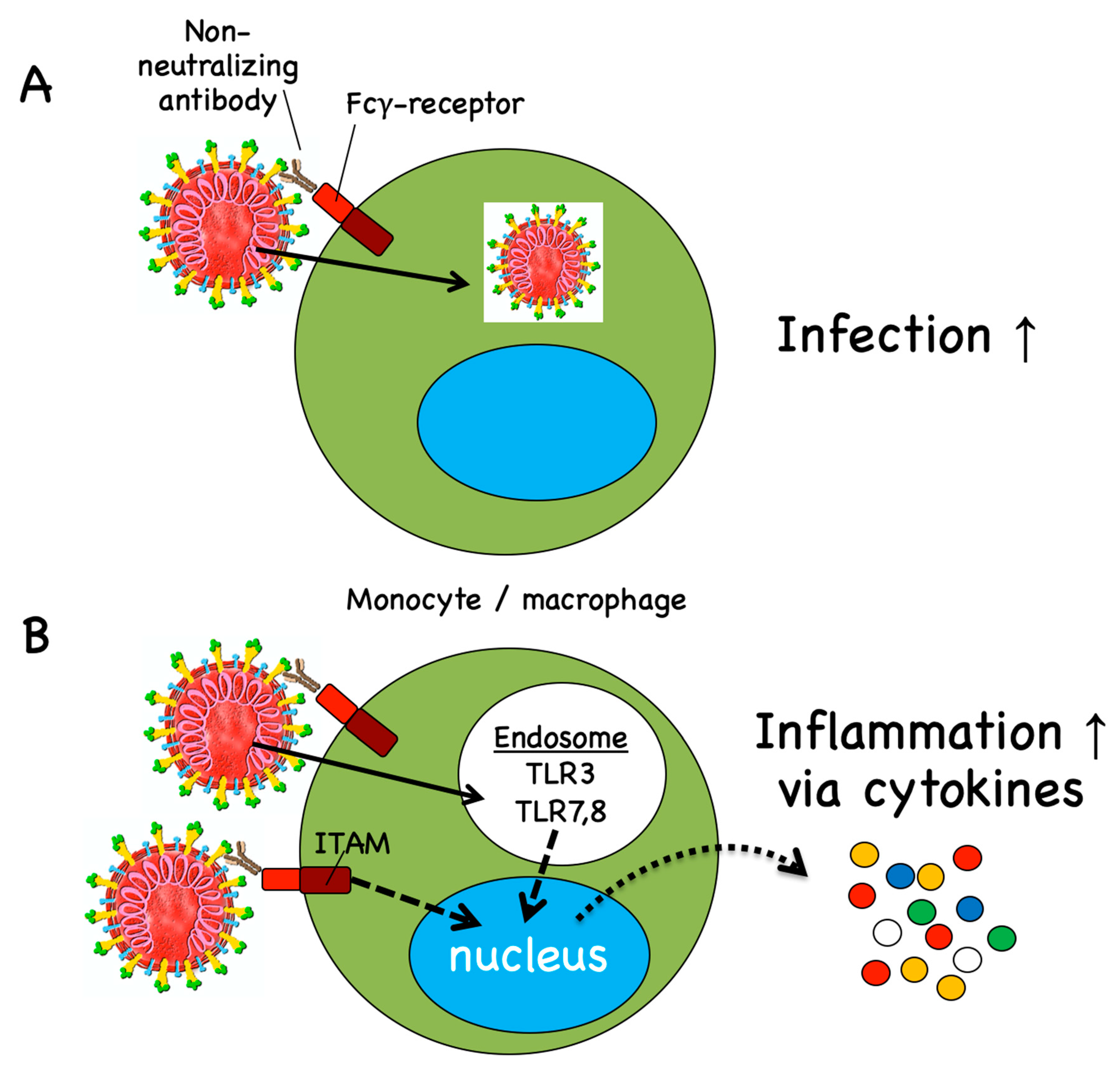
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html
Acceptance And Attitudes Toward Covid 19 Vaccines A Cross Sectional Study From Jordan
Covid 19 Vaccine Hesitancy And Resistance Correlates In A Nationally Representative Longitudinal Survey Of The Australian Population
